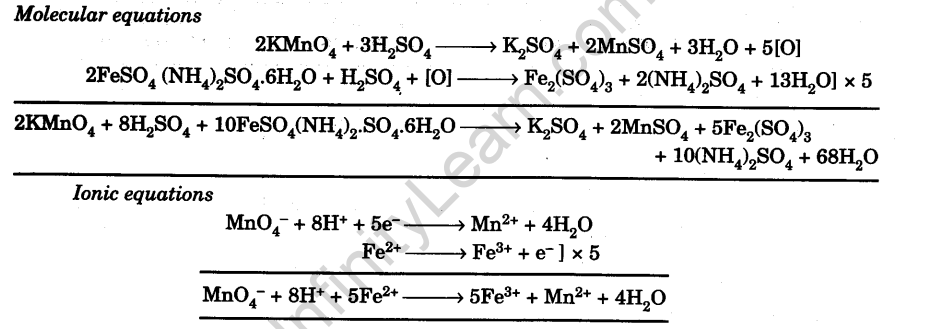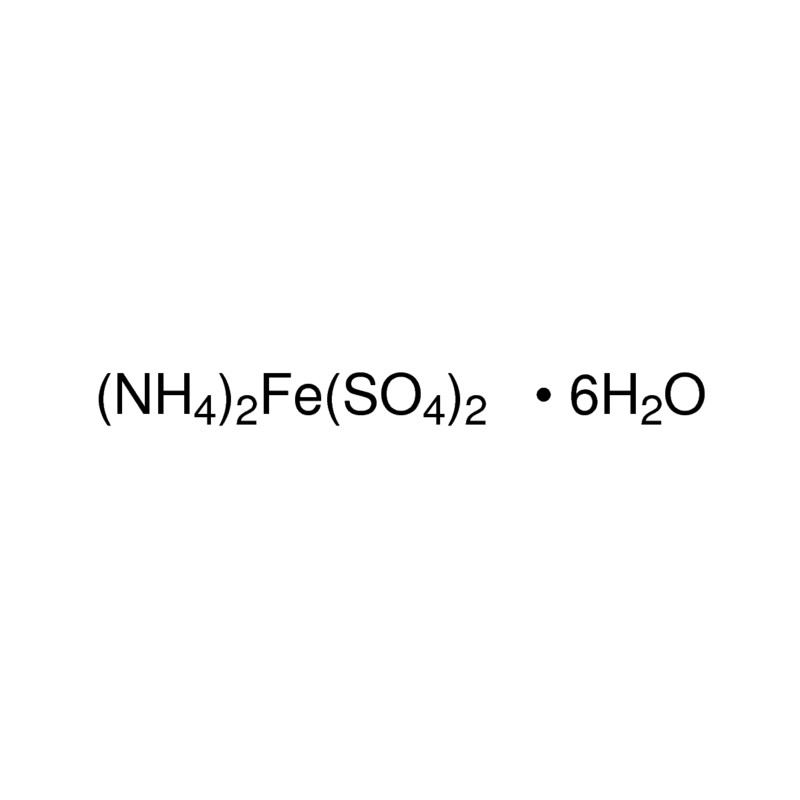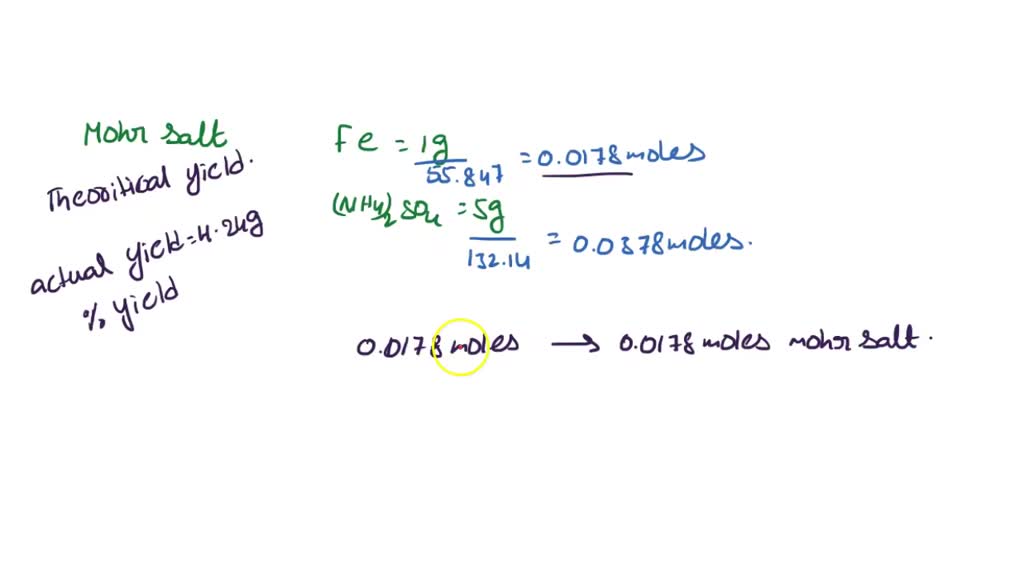
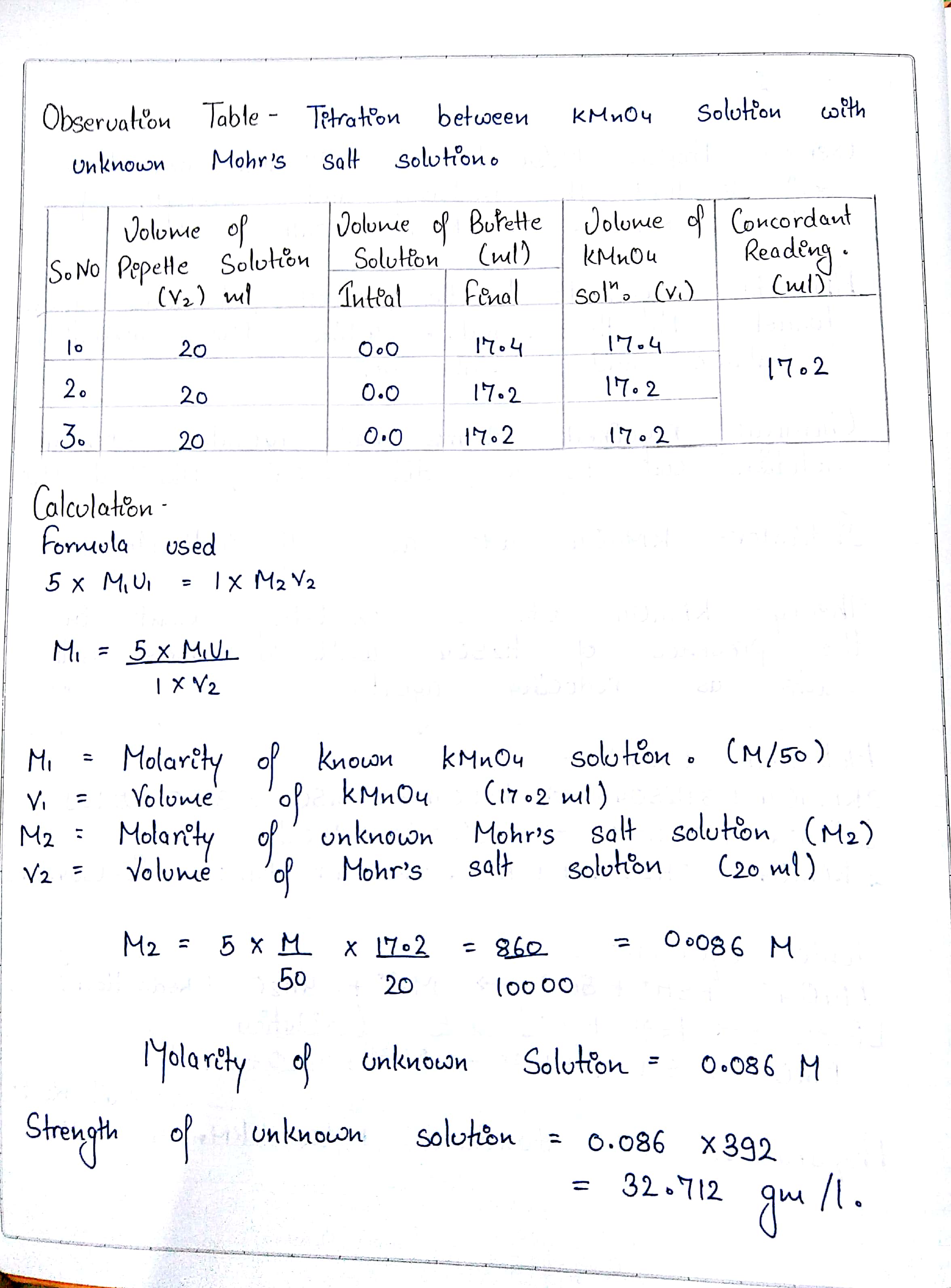
SOLVED: synthesis of Mohr's salt What is the theoretical yield of the preparation when weighing in 1 gram of iron and 5 gram of ammonium sulphate? What is the efficiency with a
Most Online Prospecting Physics | Chemistry Download List of Practicals & Experiment 2020 of CBSE RBSE NCERT Examinations

The percentage composition of Mohr's salt is 14 32% Fe2+, 9 2% NH4+, 49% SO42- 27 57% H2O - Chemistry - Some Basic Concepts of Chemistry - 10320757 | Meritnation.com

does mohr's salt contain iron ? write the chemical formula of mohr's salt? from brainly - Brainly.in

The equivalent weight of Mohr's salt, F eSO 4· N H 42 SO 4· 6 H 2 O is equal to:A. Molar weight of Mohr's saltB. One fourth of the molar weightC.