magnesium isotopes atomic structure backdrop - physics theory illustration schematic Stock Photo - Alamy
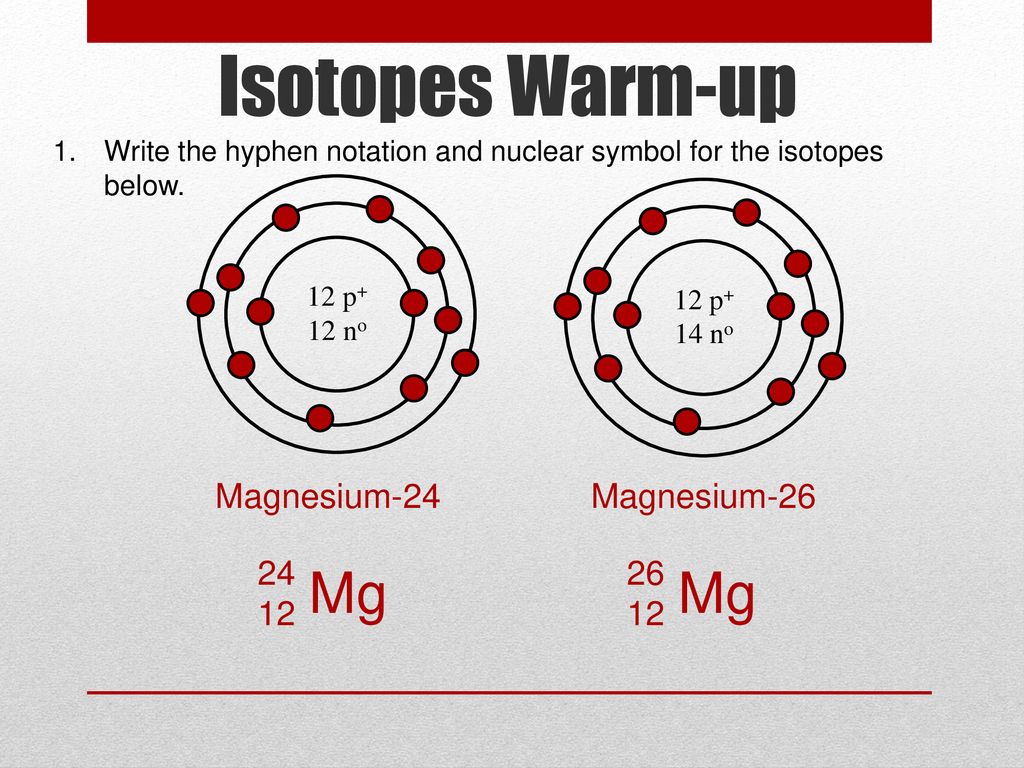
Isotopes Warm-up Write the hyphen notation and nuclear symbol for the isotopes below. 12 p+ 12 no 12 p+ 14 no 2. A atom has 26 protons and 30 neutrons. - ppt download
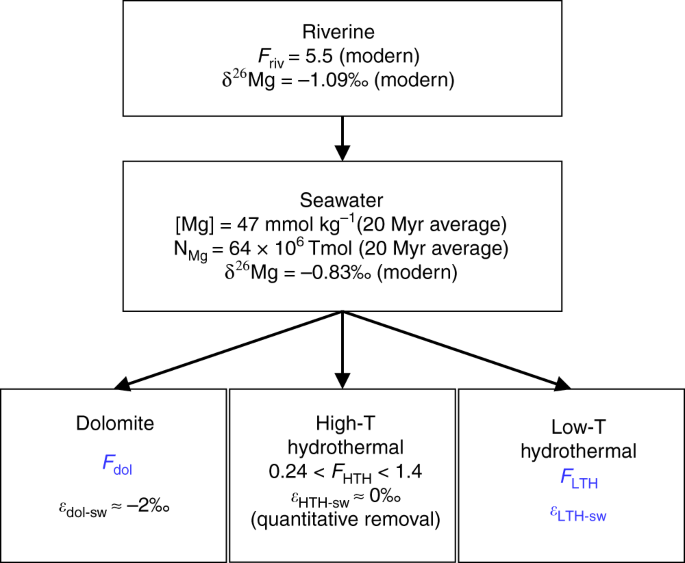
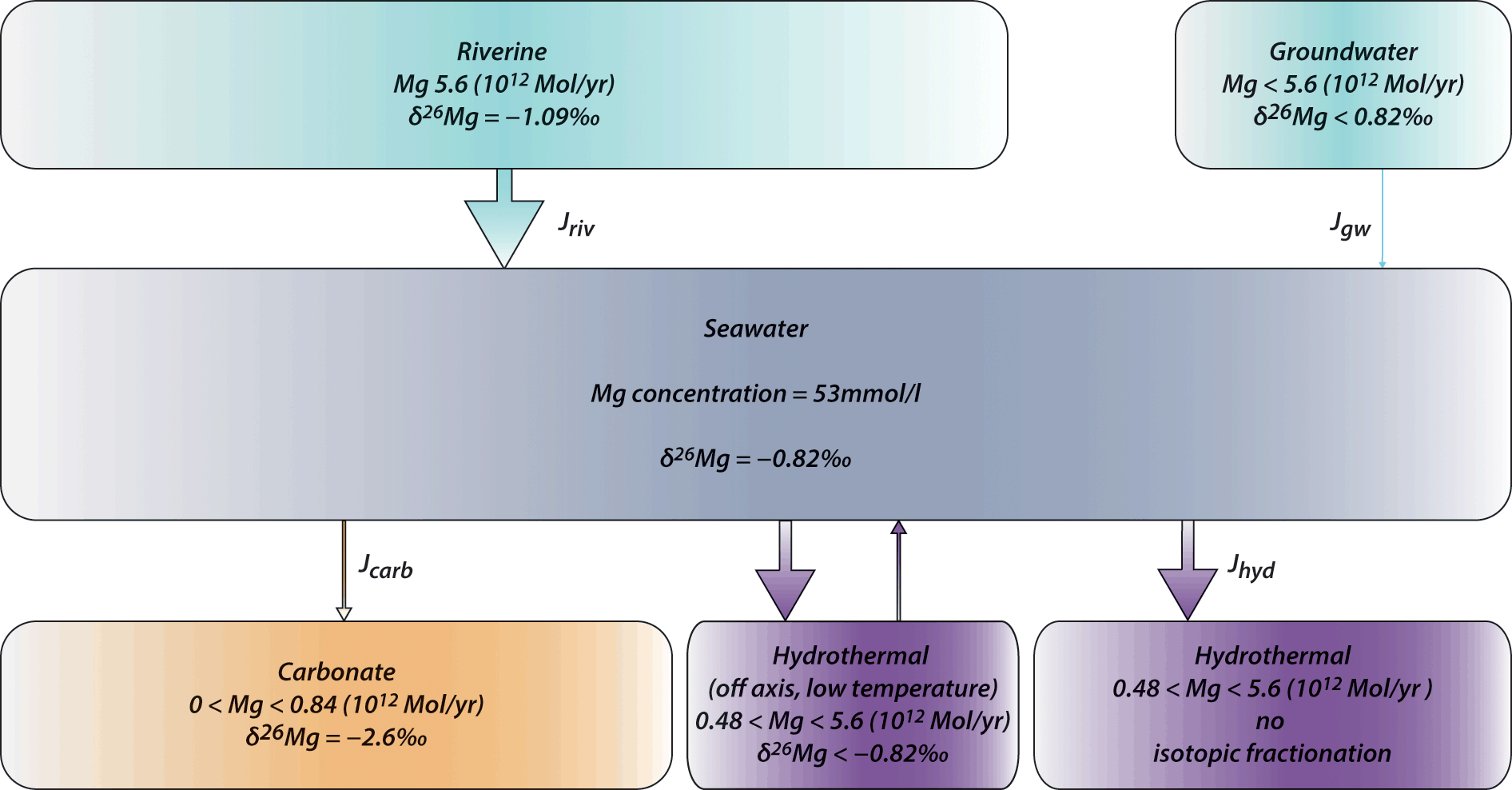
New isotope constraints on the Mg oceanic budget point to cryptic modern dolomite formation | Nature Communications

Magnesium isotopes tracer global biogeochemical cycle magnesium past and present or archive alteration | Geochemistry and environmental chemistry | Cambridge University Press

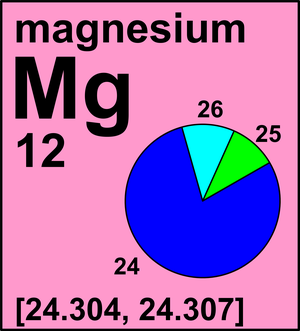
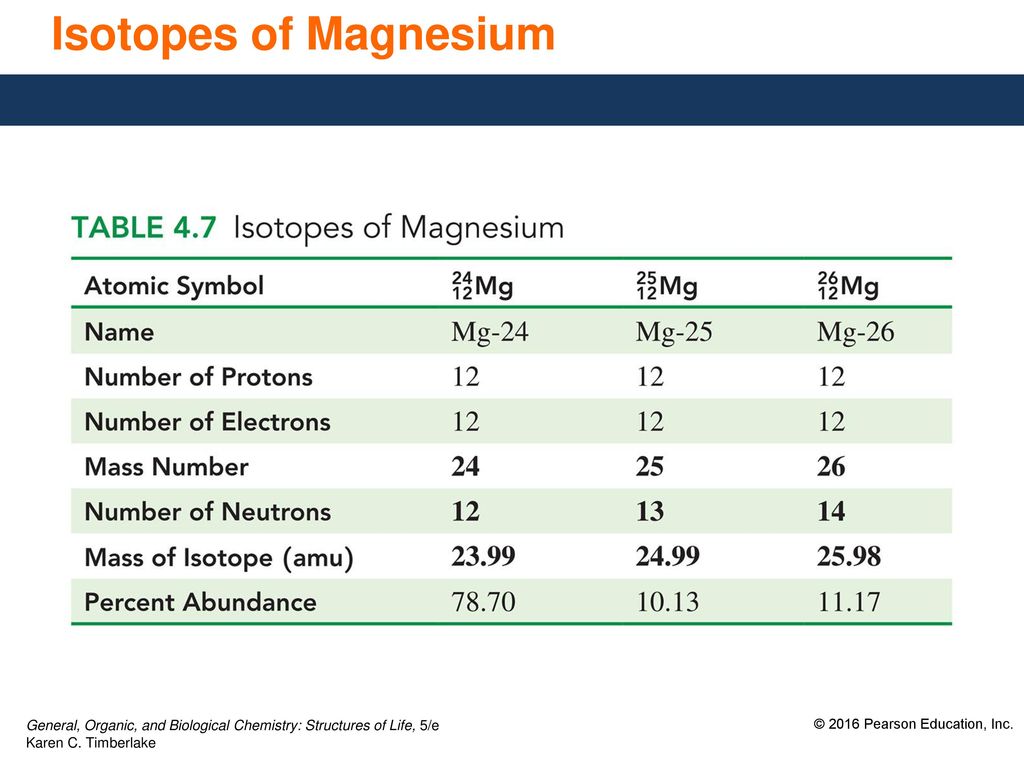
Magnesium has two isotopes ^2412Mg and ^2612Mg . According to which isotopes of magnesium have different mass numbers.

Magnesium has two isotopes ^2412Mg and ^2612Mg .Both the isotops have same electronic configurations.

magnesium isotopes atomic structure backdrop - physics theory illustration schematic Stock Photo - Alamy
magnesium isotopes atomic structure backdrop - physics theory illustration schematic Stock Photo - Alamy
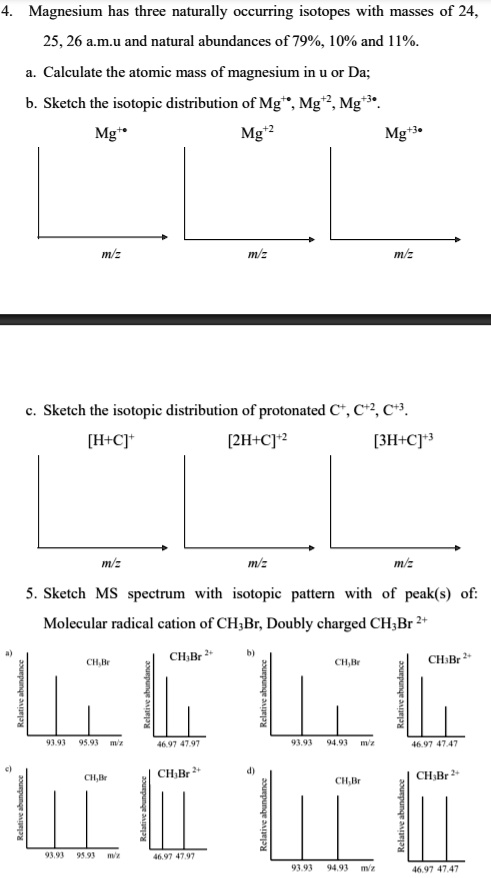
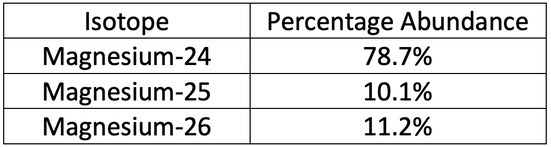
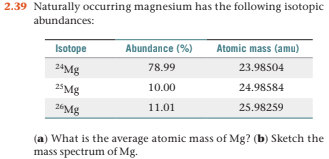
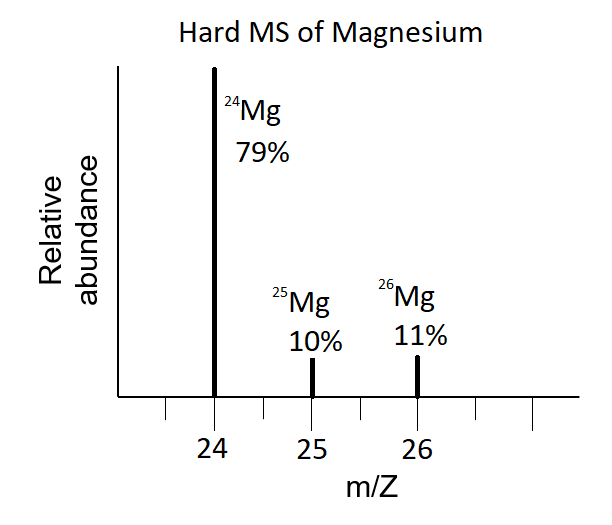
SOLVED: Magnesium has three naturally occurring isotopes with masses of 24, 25,26 a.m.u and natural abundances of 79%, 10% and 11%. Calculate the atomic mass of magnesium in U or Da; Sketch