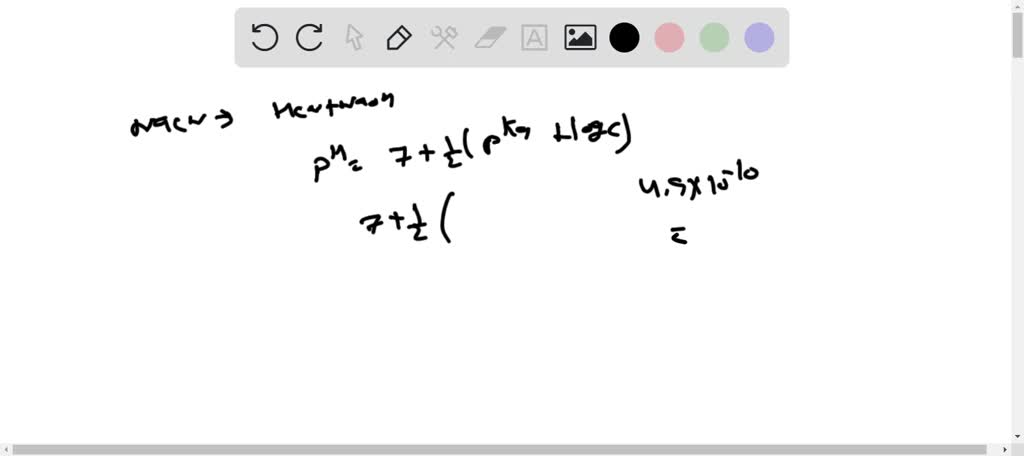
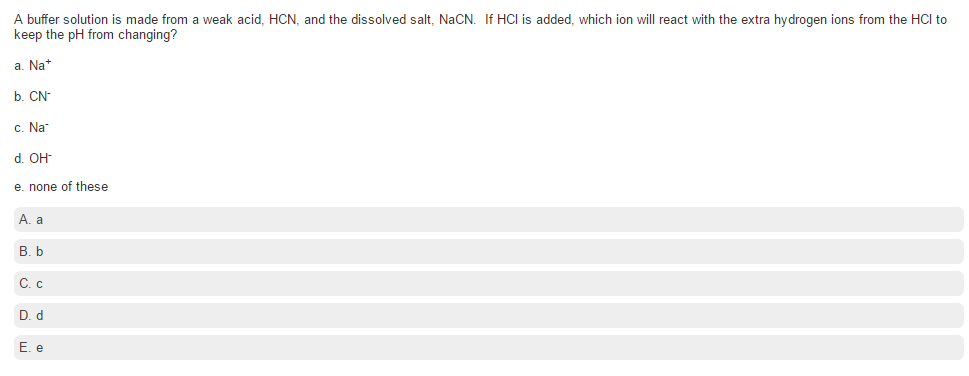
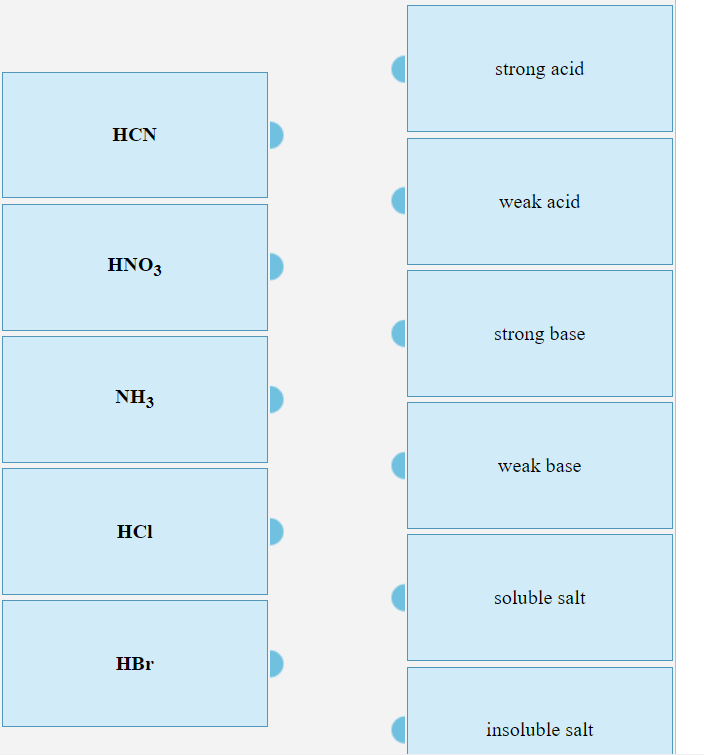
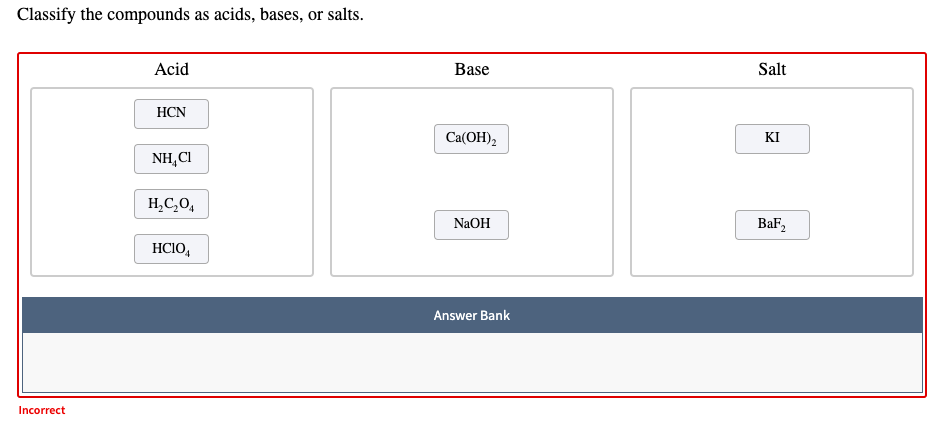
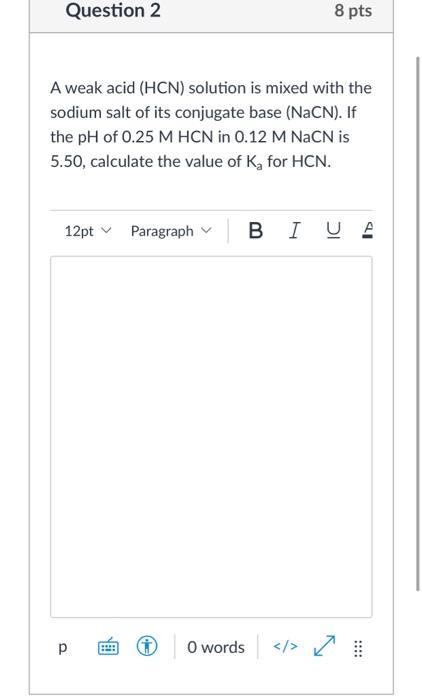
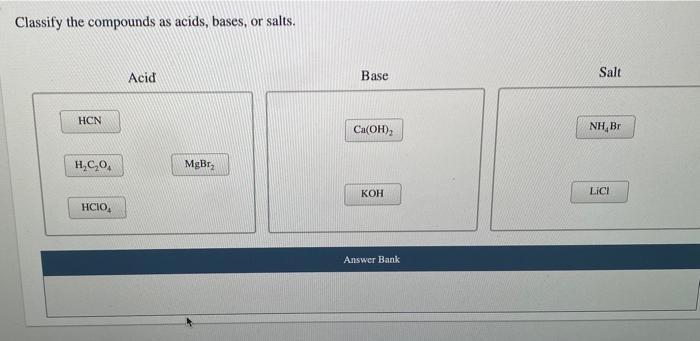
Acid-base Equilibria n K a and K b n % dissociation of weak acid/bases n pH of weak acid/base solutions n pH of salt solutions n Buffers n pH of buffer. -

Hydrogen cyanide (HCN)- Lewis acid Structure, Molecular mass, Physical and Chemical Properties, Uses with FAQs of Hydrogen Cyanide.
![Salts of HCN‐Cyanide Aggregates: [CN(HCN)2]− and [CN(HCN)3]− - Bläsing - 2020 - Angewandte Chemie International Edition - Wiley Online Library Salts of HCN‐Cyanide Aggregates: [CN(HCN)2]− and [CN(HCN)3]− - Bläsing - 2020 - Angewandte Chemie International Edition - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/ec0eebe8-512c-4b8b-a209-39a640a4e6d1/anie201915206-toc-0001-m.jpg)
Salts of HCN‐Cyanide Aggregates: [CN(HCN)2]− and [CN(HCN)3]− - Bläsing - 2020 - Angewandte Chemie International Edition - Wiley Online Library

SOLVED: Identify the salt thatis produced from the acid-base neutralization reaction between sodium hydroxide and hydrocyanic acid (HCN): sodium Cyanigc sodium amide socium formare socium acerate

1 Acid-Base Properties of a Salt Solution One of the successes of the Brønsted- Lowry concept of acids and bases was in pointing out that some ions can. - ppt download
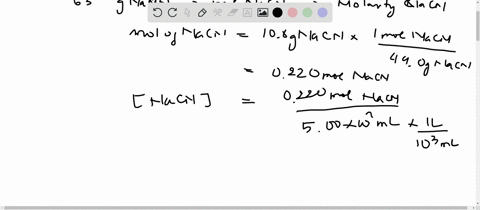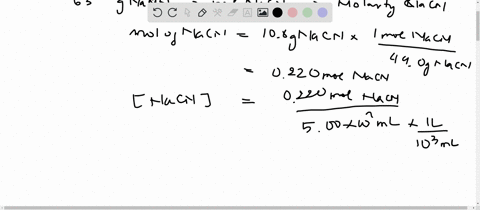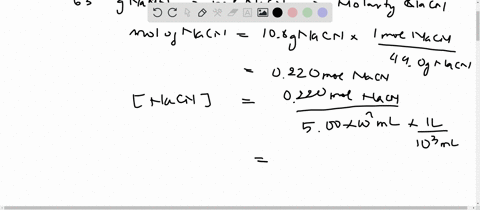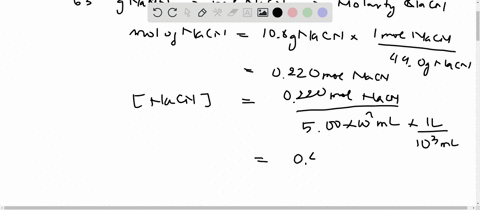
SOLVED:Sodium cyanide is the salt of the weak acid HCN. Calculate the concentrations of H3 O^+, OH^- HCN, and Na^+ in a solution prepared by dissolving 10.8 g of NaCN in enough

Effect of salt stress on HCN content (µg/g) of sorghum genotypes at 35 DAS. | Download Scientific Diagram

Effect of salt stress on HCN content (µg/g) of sorghum genotypes at 35 DAS. | Download Scientific Diagram

Ammonium cyanide is a salt of NH(4)OH(K(b)=1.8xx10^(-5)) and HCN (K(a)=4.0xx10^(-10)). The hydrolysis constant of 0.1 M NH(4)CN at 25^(@)C is :